by Preston MacDougall March 04, 2008
A limited word-count, not to mention recently weakened First Amendment protections in our country, and on university campuses in particular, will curtail explicit politico-chemical punditry. For the rest of this commentary, you're on your own.
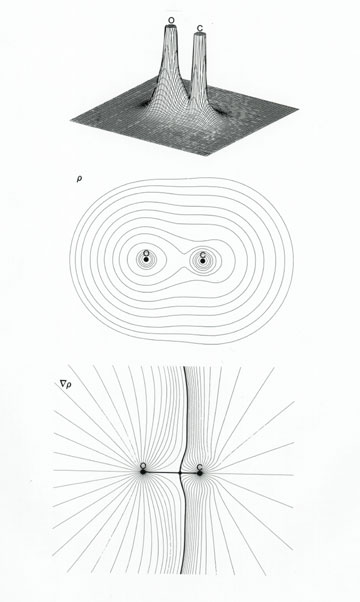
Carbon and oxygen are two of the most essential elements to life, and they can team up with other elements to form molecules of unlimited variety - from DNA to Goop. Most people know that carbon monoxide is a toxic gas, but it has its beneficial side as well - it is a key component of syngas, which is essential for converting petrochemical waste into productive energy. You can probably tell from its name that carbon monoxide is a one-on-one confrontation of carbon and oxygen atoms, and its chemical formula is indeed CO. As in any molecule, the primary bonds that hold the atoms together can be polar or non-polar, depending on the outcome of a tug-of-war over the electrons that are up for grabs between each pair of bonded atoms. In the case of CO, although there's always a little jiggling going on, the two atoms aren't much more than a tenth of a nanometer apart in their positions. Even so, the chemistry of carbon monoxide indicates that the C-O bond is polar, meaning that the bonding electrons have a definite preference for one atom over the other. But which one? You might think that advances in computer modeling of complex systems, such as fantasy football, would make such a basic question about a single, simple molecule seem almost trivial. But a wide range of answers are to be found among chemical pundits using assorted Chemical Modeling Methods, or CMM. Legendary among chemical pundits was the great Linus Pauling - the only person to win two unshared Nobel Prizes, and who probably would have won a third (though not unshared), for solving the structure of DNA, if the U.S. State Department hadn't limited his international travel during the Cold War. In his classic treatise on "The Nature of the Chemical Bond", on the page devoted to carbon monoxide, he anticipates the reader's dissatisfaction with his best answer that four bonding situations are about equally probable - two non-polar, and two that are polar, but in opposite senses. He does note, however, that, while other factors come into play, "the great electronegativity of oxygen" favors primary bonding that gives oxygen the edge in electron count. Electronegativity is a concept that Pauling himself had introduced into the lexicon of chemical punditry. It essentially reflects an atom's ability to draw electrons toward itself, and away from other atoms. It is the closest thing to "charisma" in the atomic world, and oxygen has the second highest value among all elements - a full point above carbon on a 4.0 scale. Pauling did most of his chemical punditry without the aid of computers. After computers revolutionized CMM, new strategies for polling electron preferences became fashionable, particularly the so-called "Mulliken population analysis". The problem with this technique is that it doesn't actually consider where electrons are likely to be positioned within the molecule, but only the "fit" between electron preferences and arbitrary questions that are posed by the computer and are meant to represent each atom. It is up to the chemical pundit to compile the list of questions, called the "atomic basis set". I have over twenty years of experience with CMM. After studying this computational technique closely, I found that, by carefully designing the list of questions, I could get a statistically satisfactory model of the molecule overall, but any value I wanted for the polarity of the bond. When only the actual positions of electrons are considered, which is the method that I prefer, the clear preference of electrons in carbon monoxide is for the oxygen atom. While I hope that it will become conventional for others to regard the polarity of the C-O bond as I do, there is the possibility that disagreements will persist and there will be no consensus. This could hamper much-needed efforts to engineer optimal processes for converting petrochemical waste into productive energy. In a worst-case scenario, frustrated chemists could suggest that since carbon monoxide is one of many greenhouse gases, we should ignore any overall benefits and look elsewhere for solutions to national problems involving waste and energy. With the atomic symbol Al, the element aluminum comes to mind when one thinks of reducing greenhouse gases, but its electronegativity is another full point below carbon's.
On the Web:
E-mail your letters & opinions to editor@sitnews.us SitNews ©2007 Stories In The News Ketchikan, Alaska |
||